The hard facts about hard water
The hard facts about hard water: How removal of mineral soils improves the comfort and care of textiles.
Published
November 18, 2021
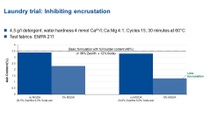
The key to softer, cleaner laundry is controlling the minerals in the water. Fabrics become encrusted during the laundry process by deposits from hardness in water or from the selected detergent. For example, sodium carbonate used in some detergents can react with soluble minerals in water, creating calcium carbonate and encrusting the fabric.Fabric fibers that normally lay down smoothly next to one another, when encrusted with calcium carbonate (CaCO3) become brittle and rough. Polymers such as Sokalan® PA and Sokalan CP can provide a considerable reduction in encrustation.

Like chelating agents, Sokalan® polymers work as scale inhibitors but differently: they prevent the growth of crystals into a scale, maintaining the microscopic crystals dispersed and preventing them from depositing onto fabrics.

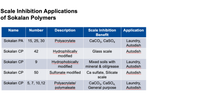
To get the best scale inhibition, it is important to match the type of salt present in the water with the most effective polymer at preventing scale formation of that specific compound. Sokalan® PA (15, 25, 30) and Sokalan CP (5, 7, 10, 12) produce the best result when dealing with calcium carbonate (CaCO3) and calcium sulfate (CaSO4). When you have a silicate-type scale, Sokalan CP 50 or Sokalan CP 42 performs best. Nearly all Sokalan products have some anti-scale benefit.